Medical Rapid Diagnosis hepatitis e (hev) virus igm test
- US $0.48
1,000 - 4,999 piece
- US $0.42
5,000 - 19,999 piece
- US $0.40
20,000 - 799,999 piece
- US $0.32
800,000 - 999,999 piece
group nameHepatitis testing series
-
Min Order1000 piece
brand nameVoyage
modelcassette 3.0mm
payment methodL/C, Western Union, T/T, Paypal
-
update timeFri, 16 Aug 2019 09:56:36 GMT
Paramtents
Place of Origin Jiangsu, China (Mainland)
Model Number cassette
Specimen serum/plasma
Valid 24 months
Oem Service available
storage room temperature
Accuracy over 99%
Size 3.0 mm
Packging & Delivery
Size12cm x 6cm x 1cm
Weight0.01kg / piece
Min Order1000 piece
Briefing
Detailed
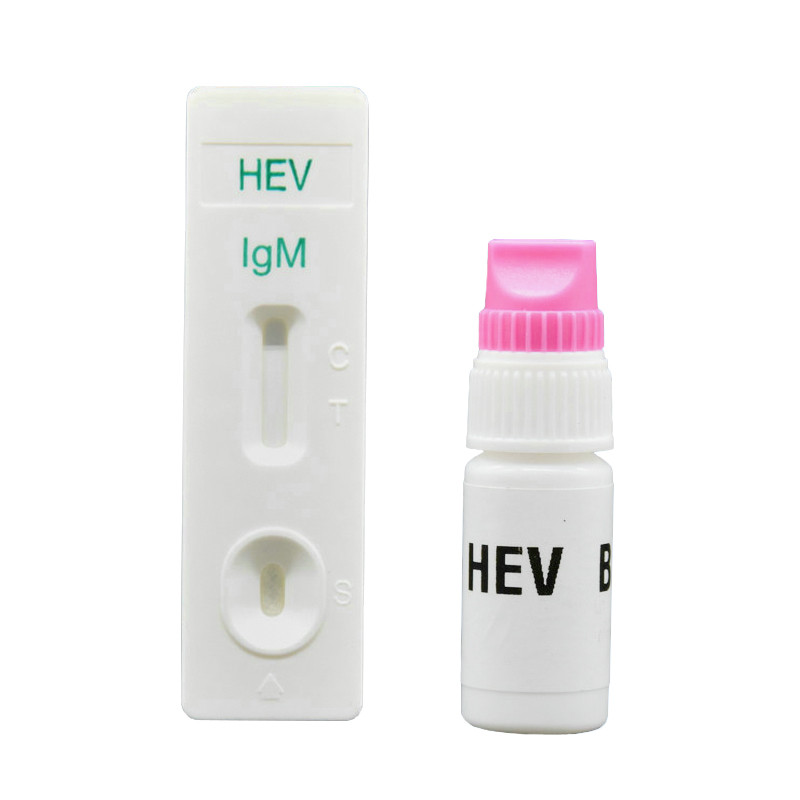
Intended use:
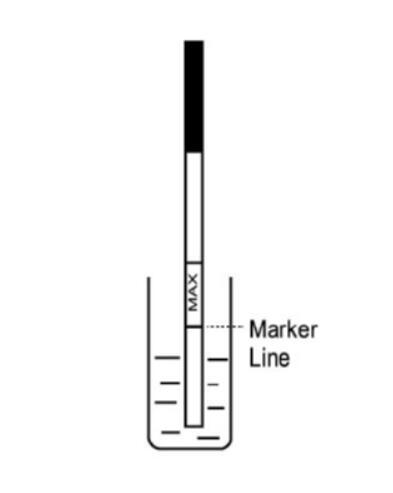
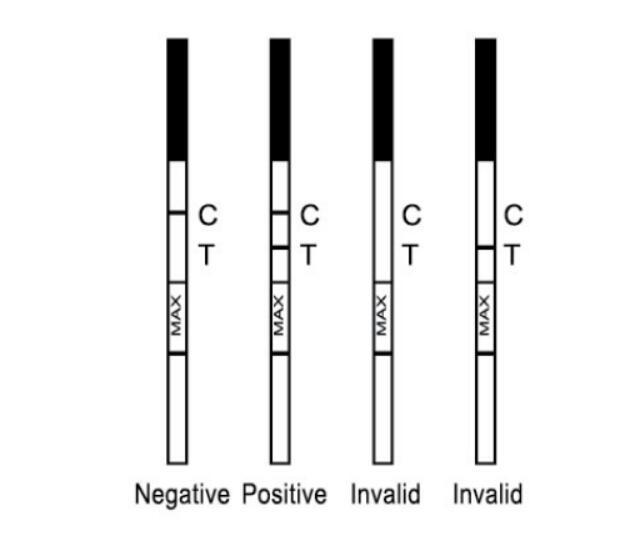
You need a product
Related Searches
You May Like
6YRS Nantong Voyage International Trade Co., Ltd.
- Delivery clauses under the trade mode
- FOB, CFR, CIF, EXW, Express Delivery
- Acceptable payment methods
- T/T, L/C, Westem Union