COVID-19 Antigen Neo P211136 20Tests
group namekit swab samples
-
Min Order100 piece
modelP211136
payment methodL/C, D/A, D/P, Western Union, MoneyGram, T/T, Paypal
-
update timeTue, 06 Jul 2021 09:55:11 GMT
Paramtents
CE certificate No NLCA002202049626
Specimen Whole blood/Plasma/Serum
Material Plastic
Packging & Delivery
Min Order100 piece
Briefing
Detailed


|
CONTENTS
|
|||
|
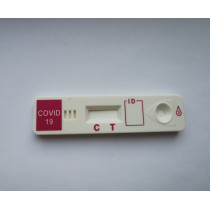
COVID-19 antigen test devices
|
20 tests
|
||
|
Sterilized swabs
|
20 pcs.
|
||
|
Extraction buffer, 7mL
|
2 pcs.
|
||
|
Nozzles with filter
|
20 pcs.
|
||
Plastic tubes | 20 pcs. | ||
Tube Stand | 1 pcs. | ||
Package Insert | 1 pcs. | ||